The majority of catalytic methods for the stereoselective formation of new C–C and C–heteroatom bonds involve a single activation mode, such as Lewis base or acid catalysis. N-Heterocyclic carbenes (NHCs) are versatile Lewis bases that catalyze a variety of bond-forming processes, including formal [m+n] cycloadditions. One drawback of these formal cycloadditions is that the electrophilic substrates are limited to reactive C=X π systems, such as aldehydes and imines. The use of a second mode of activation to produce a more reactive electrophile in situ, is one strategy to address this problem.
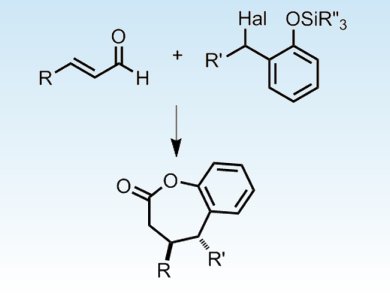
Karl Scheidt and co-workers, Northwestern University, USA, combined two distinct Lewis base activation modes to achieve an enantioselective organocatalytic formal [4+3] heterocycloaddition. This dual activation strategy involved the formation of two transient species, a nucleophilic NHC homoenolate and an electrophilic o-quinone methide, thus expanding the scope of the electrophile beyond C=X π systems.
They used this method to synthesize 2-benzoxopinones, which are found in natural products and as the core of many biologically active small molecules.
- A Dual Lewis Base Activation Strategy for Enantioselective Carbene-Catalyzed Annulations,
Javier Izquierdo, Ane Orue, Karl A. Scheidt,
J. Am. Chem. Soc. 2013.
DOI: 10.1021/ja405833m