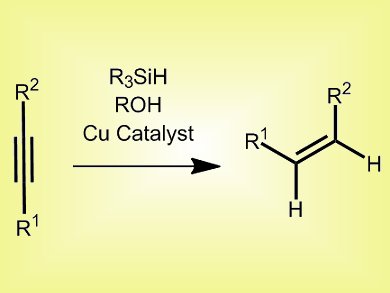
The semireduction of alkynes to alkenes is an important synthetic transformation. General catalysts, e.g., Lindlar and P2 nickel, are often non-specific, resulting in isomerization or over-reduction to the alkane; and specific catalysts tend to be scope limited. Therefore, a general method for specific semireduction is needed.
Aaron Whittaker and Gojko Lalic, University of Washington, USA, have developed a copper catalyst system, which uses a copper alkoxide with a bulky IPr ligand (1,3-bis-(2,6-diisopropylphenyl)imidazol-2-ylidene) attached, polymethylhydrosiloxane, and isobutyl alcohol. The mechanism involves copper hydride formation, followed by hydrocupration of the alkyne and protonation of the alkenyl-copper intermediate to form the desired product.
This system benefits from readily-available starting materials, low catalyst loading (0.5 mol-%), low temperatures (25–45 °C), short reaction times (1 h) and excellent chemoselectivity, and works for both internal and terminal alkynes in the presence of many functional groups, including nitroarenes and aryl halides.
- Monophasic Catalytic System for the Selective Semireduction of Alkynes,
A. M. Whittaker, G. Lalic
Org. Lett. 2013.
DOI: 10.1021/ol4001679