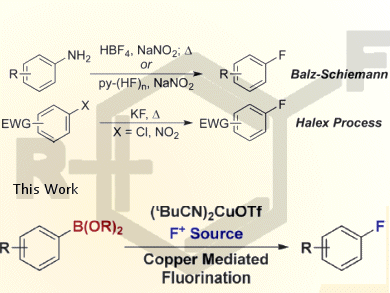
To overcome the limitations of classical methods for the synthesis of aryl fluorides by the Halex (halogen exchange) or Balz−Schieman reactions, John F. Hartwig, University of California, Berkeley, USA, and colleagues reported the direct conversion of arylboronate esters to aryl fluorides under mild conditions with readily available reagents. They also developed tandem reactions for the fluorination of arenes and aryl bromides through arylboronate ester intermediates.
The fluorination reaction occurs through facile oxidation of Cu(I) to Cu(III). It is followed by rate-limiting transmetalation of a bound arylboronate to Cu(III). Fast C−F reductive elimination is proposed to occur from an aryl−copper(III)−fluoride complex.
Stoichiometric reactions of this Cu(III) species show that it is competent to be an intermediate in the fluorination process.
- Copper-Mediated Fluorination of Arylboronate Esters. Identification of a Copper(III) Fluoride Complex,
Patrick S. Fier, Jingwei Luo, John F. Hartwig,
J. Am. Chem. Soc. 2013.
DOI: 10.1021/ja310909q