Global production of methyl methacrylate was 4 million metric tons in 2010. Methyl methacrylate is a monomer used in the large-scale manufacturing of lightweight, shatter-resistant alternatives to glass such as Plexiglas. Each kilogram of methyl methacrylate produced, yields 2.5 kg of ammonium hydrogen sulfate, a corrosive byproduct that is not usable. Disposal of ammonium hydrogen sulfate is extremely energy intensive.
David Tyler and colleagues, University of Oregon (UO), USA, have identified a catalyst that could reduce the amount of ammonium hydrogen sulfate. The catalyst is a metal nanoparticle that creates an active site at which the starting material acetone cyanohydrin can be converted into the methacrylate precursor, according to the UO.
The next step will be to speed up the conversion of methyl methacrylate, reducing the process to the span of a minute or two, to meet the industrial standard of a productive catalyst.
- Presented Tuesday, Aug. 21, at the national meeting of the American Chemical Society in Philadelphia, USA
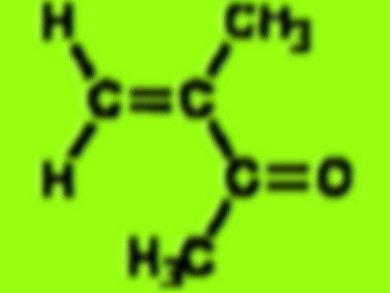




according to this molecule function as catalysis for the glass such as flexiglas, could you tell me the benefit of the reactions between catalysis and substrat enzime (you told us its an active site) ,for this material … then may i get the journal for free ..? thanks [email protected]