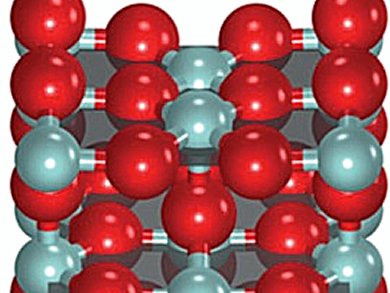
In heterogeneous catalysis, rates with Arrhenius-like temperature dependence are ubiquitous. Also common are compensation phenomena, which arise from the linear correlation between the apparent activation energy and the logarithm of the apparent pre-exponential factor. This phenomenon of compensation in heterogeneous catalysis was described by F. H. Constable and Erika Cremer 1925, but no direct experiments had substantiated these theoretical insights.
Detre Teschner, Fritz-Haber Institute of the Max Planck Society, Berlin, Germany, Núria López, Institute of Chemical Research of Catalonia (ICIQ), Tarragona, Spain, and colleagues studied the origin of compensation and found a similar dependence on the rate-limiting surface coverage term for each Arrhenius parameter. This result is derived from an experimental determination of the surface coverage of oxygen and chlorine species using temporal analysis of products and prompt gamma activation analysis during HCl oxidation to Cl2 on a RuO2 catalyst. This was also substantiated by theory.
The compensation phenomena was found to appear when the effect on the apparent activation energy caused by changes in surface coverage is balanced out by the entropic configuration contributions of the surface.
This result sets a new paradigm in understanding the interplay of compensation effects with the kinetics of heterogeneously catalysed processes.
- In situ surface coverage analysis of RuO2-catalysed HCl oxidation reveals the entropic origin of compensation in heterogeneous catalysis,
Detre Teschner, Gerard Novell-Leruth, Ramzi Farra, Axel Knop-Gericke, Robert Schlögl, László Szentmiklósi, Miguel González Hevia, Hary Soerijanto, Reinhard Schomäcker, Javier Pérez-Ramírez, Núria López,
Nature Chem. 2012.
DOI: 10.1038/nchem.1411 - Videos on Surface Configurational Entropy:
http://www.nature.com/nchem/journal/vaop/ncurrent/extref/nchem.1411-s2.mp4
http://www.nature.com/nchem/journal/vaop/ncurrent/extref/nchem.1411-s3.mp4