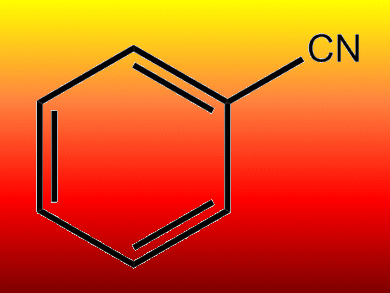
Cyanation is an important tool for the introduction of various functionalities such as carboxylic acids, aldehydes, ketones, oximes, amides, amines, azoles, and oxazolidines in aromatic and heteroaromatic rings. Most protocols for the production of aryl nitriles require highly toxic and environmentally hazardous cyanide sources such as KCN, NaCN, or CuCN.
Bhalchandra Bhanage and co-workers, Deemed University, Mumbai, India, have sought an alternative route to nitriles that is cyanide-free. Their single step protocol uses formamide as both a cyanide source and solvent, with a palladium acetate/xantphos catalyst and phosphorus oxychloride.
A range of aryl bromides and iodides could be converted into aryl nitrides with up to 93 % yield. Treatment of diiodobenzene with formamide obtained phthalimide instead of dinitrile under these conditions, giving the first example of a CO-free synthesis of phthalimide from diiodobenzene.

- Cyanides-Free Cyanation of Aryl Halides using Formamide
D. N. Sawant, Y. S. Wagh, P. J. Tambade, K. D. Bhatte, B. M. Bhanagea,
Adv. Synth. Catal. 2011.
DOI: 10.1002/adsc.201000807


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
