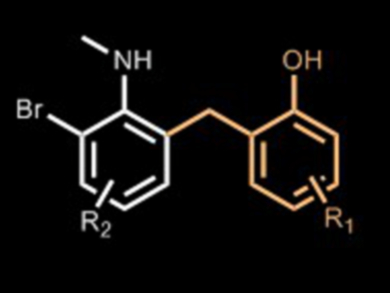
Benzyl-substituted anilines are useful target molecules for organic synthesis. Previously developed approaches to these substrates include hydroarylation, Pd- or Ni-catalyzed cross-coupling, and the traditional Friedel-Crafts reaction. However, there is still room for alternative preparation methods, especially taking into account chemo- and regioselectivity.
Manuel Amézquita-Valencia and Howard Alper, University of Ottawa, Canada, have established a synthesis of benzyl-substituted anilines involving a Pd-catalyzed rearrangement under CO pressure. The team used 2-[{(2-bromophenyl)methylamino}methyl]phenol as the model substrate, Pd(OAc)2 as the catalyst, and K3PO4 as a base under 300 psi CO in CH2Cl2 solvent, all of which provided 83 % isolated yield of the desired rearranged aniline after 15 h at 120 °C.
The reaction proved tolerant of halide and alkyl substituents on the aryl moieties. The selectivity of the process can be controlled by the amino substitution pattern at the arene. According to the researchers, the high regioselectivity of the process makes it a useful synthetic tool.
- Palladium-Catalyzed Regioselective C-Benzylation via a Rearrangement Reaction: Access to Benzyl-Substituted Anilines,
Manuel Amézquita-Valencia, Howard Alper,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201603941