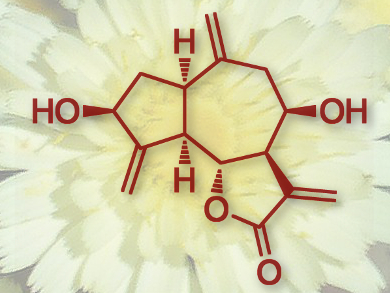
A compound found in the Mediterranean plant Andryala intergrifolia, (±)-integrifolin, has a potent biological effect: It inhibits the proliferation of human cancer cell lines. In addition to its biological functions, it has an interesting molecular structure, with three exo-methylene moieties and six asymmetric centers on a bicyclo[5.3.0]decane skeleton (pictured).
Nobuharu Iwasawa, Tokyo Institute of Technology, Japan, and colleagues have developed the first total synthesis for the compound. A key step is a cleverly designed trienyne cyclization using a tungsten catalyst, which delivered the desired bicyclic intermediate in high yield and as a single stereoisomer. This intermediate allowed further ring modification and construction of the desired final product, integrifolin, over a total of 27 synthetic steps and in an overall yield of 0.23 %.
The work provides a promising cyclization strategy for the construction of complex polycyclic molecules and could be a basis for future development in this area.
- Total Synthesis of (±)-Integrifolin,
Katsuya Shimomaki, Hiroyuki Kusama, Nobuharu Iwasawa,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201601275