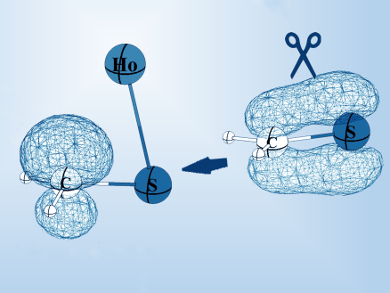
Bare holmium(I)ions are known for being quite unreactive. However, Helmut Schwarz and colleagues, Technical University Berlin, Germany, found a reaction in which holmium does function in the activation of toluene. The team investigated the thermal reaction of [Ho(CH2S)]+ with toluene to produce [C6H5CHSHo]+ and CH4 using Fourier-transform ion cyclotron resonance mass spectrometry (FT-ICR MS), complemented by density functional theory (DFT) calculations.
According to the researchers, the Ho+ ion converts the closed-shell substrate CH2S to a “prepared” state ([Ho(CH2S)]+). It possesses a carbon radical and is then capable of hydrogen-atom transfer from toluene. Using this approach, a sequence of hydrogen-atom abstractions combined with the making and breaking of C‒S, S‒Ho, and C‒H bonds are permitted.
- Mechanistic Aspects of the Holmium-Mediated, Reciprocal Hydrogen/Sulfur Exchange in the Gas Phase: C6H5CH3 + CH2S → C6H5CHS + CH4,
Shaodong Zhou, Jilai Li, Maria Schlangen, Helmut Schwarz,
Chem. Eur. J. 2016, 22, 4336–4339.
DOI: 10.1002/chem.201600061