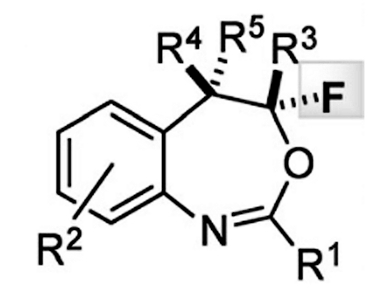
Fluorinated organic compounds are of great interest to any number of fields of chemistry, including pharmaceutical and agrochemical industry, non-invasive diagnosis, and development of “intelligent” materials. The presence of a fluorine atom can have a significant impact on a molecule’s solubility, bioavailability, and stability.
Tanja Gulder and co-workers at TU Munich in Germany have reported the synthesis of the pharmacologically interesting heterocycles 4-fluoro-1,3-benzoxazepines from o-styryl benzamides by using a fluorination/aryl Migration/cyclization cascade strategy. Notably, Gulder’s synthesis avoid the need for transition metal catalysts, instead using a bench-stable hypervalent fluoro iodane reagent as an electrophilic source of fluorine. This reagent is not only significantly more reactive than the well-established iodine(III)-based fluorinating reagent Selectfluor, but provides completely different chemoselectivity, providing seven-membered ring benzoxazepines instead of oxazines. Moreover, this strategy is used in the synthesis of 20 structurally distinct congeners and proceeds with complete regioselectivity under mild reaction conditions.
- A Fluorination / Aryl Migration / Cyclization Cascade for the Metal-free Synthesis of Fluoro Benzoxazepines,
Anna Ulmer, Christoph Brunner, Andreas M. Arnold, Alexander Pöthig, Tanja Gulder,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201504749
This article is soon to feature in Chemistry ‒ A European Journal’s up-coming “Women in Chemistry” special issue.