Partial oxidation of hydrocarbons has long proven a challenging aim in catalysis, as the ligands of metal complex catalysts are themselves often subject to oxidation by active oxygen species. Biological systems overcome this problem by embedding the active center of cytochrome P-450 within the pore wall of a protein.
With this in mind, Atsushi Fukuoka and colleagues. Hokkaido University, Toyota Central R&D Laboratories, and Tokyo Institute of Technology, Japan, expected that immobilization of metal complex catalysts on the pore walls of periodic mesoporous silica (PMO) would also suppress self-oxidation, thus yielding a reusable catalyst for selective oxidation of hydrocarbons.
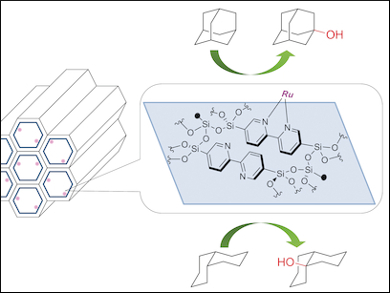
By incorporating 2,2’-bipyridyl (BPy) groups in PMO to form BPy-PMO, the team could immobilize [RuCl2(CO)n] to afford a single-site heterogeneous catalyst in which each Ru center was bound by the chelating BPy units in the BPy-PMO pore walls. With NaClO as an oxidant, the Ru catalyst was shown to selectively oxidize tertiary C-H bonds in adamantane and decalin (with perfect retention of its configuration) to the corresponding alcohols, with no loss of activity or selectivity on catalyst recycling.
- Ruthenium-Immobilized Periodic Mesoporous Organosilica: Synthesis, Characterization, and Catalytic Application for Selective Oxidation of Alkanes,
Nobuhiro Ishito, Hirokazu Kobayashi, Kiyotaka Nakajima, Yoshifumi Maegawa, Shinji Inagaki, Kenji Hara, Atsushi Fukuoka,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201502638