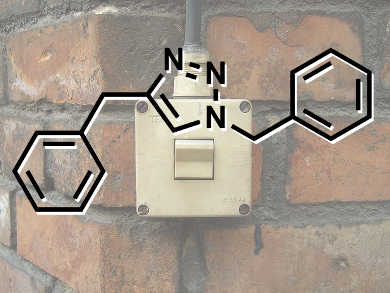
Inspired by biological molecular machines, the development of synthetic nanotechnologies that exploit controlled molecular level motion is on the rise. James Crowley and colleagues at the University of Otago, New Zealand, have shown that simple palladium(II) pyridine-2,6-carboxamide complexes coordinated to 1,2,3-triazole “click” ligands will undergo stimuli responsive ligand exchange reactions with 4-dimethylaminopyridine.
Treatment of the palladium(II) pyridine-2,6-carboxamide “click” complexes with the more basic 4-dimethylaminopyridine (DMAP) ligand leads to the complete displacement of the 1,2,3-triazole unit from the palladium complex. Addition of a stoichiometric amount of acid causes protonation of the DMAP and quantitative formation of the palladium(II) “click” complex. Upon neutralizing the acidic solution with NaOH, the palladium(II) DMAP complex is reformed.
As 1,2,3-triazole ligands can be readily synthesized using the copper(I)-catalyzed azide and alkyne cycloaddition (CuAAC) “click” reaction, the researchers believe that this acid-base driven switching mechanism could potentially be exploited to generate a wide variety of pH-responsive nanomachines.
- Acid-Base Driven Ligand Exchange with Palladium(II) “Click” Complexes,
Asif Noor, Daniel L. Maloney, James E. M. Lewis, Warrick K. C. Lo, James D. Crowley,
Asian J. Org. Chem. 2014.
DOI: 10.1002/ajoc.201402197