Atom-transfer radical addition (ATRA) reactions are effective methods for the functionalization of olefins. These reactions take place through a radical chain propagation mechanism, and as such need stoichiometric amounts of initiators, which can be toxic or explosive, and high reaction temperatures.
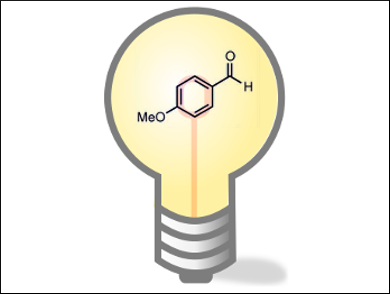
Paolo Melchiorre and colleagues, Institució Catalana de Recerca i Estudis Avançats (ICREA), Barcelona, Spain, and Institute of Chemical Research of Catalonia (ICIQ), Tarragona, Spain, have developed a method that overcomes the problems of using toxic or expensive reagents. They used molecules based on the p-anisaldehyde substrate, which, when irradiated with a standard household light bulb, can catalyze the ATRA of alkyl halide onto olefins in the presence of a base under ambient conditions. The team showed that upon excitation, the singlet state of the catalyst is formed, which undergoes intersystem crossing to the triplet state. This state sensitizes the triplet state of the alkyl halide and results in the homolytic cleavage of the C–X bond.
This process, which involves the use of a simple light bulb for triplet sensitization, represents a new advance in sustainable, environmentally friendly radical reactions.
- Photo-Organocatalysis of Atom-Transfer Radical Additions to Alkenes,
Elena Arceo, Elisa Montroni, Paolo Melchiorre,
Angew. Chem. Int. Ed. 2014.
DOI: 10.1002/anie.201406450