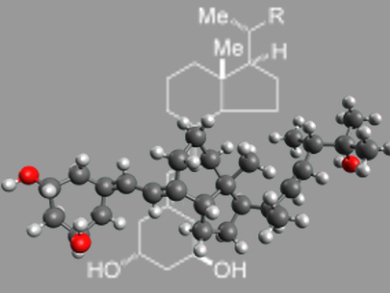
Paricalcitol, an A-ring-modified 19-nor analogue of 1α,25-dihydroxyvitamin D2, is currently used for the treatment and prevention of secondary hyperparathyroidism associated with chronic renal failure.
Kazuo Nagasawa and co-workers, Tokyo University of Agriculture and Technology, Japan, have developed a novel synthetic method for producing the A-ring synthons for paricalcitol. The synthesis is based on ring-closing olefin metathesis of a linear precursor, followed by palladium-catalyzed isomerization of the endocyclc olefin product to give exocyclic olefin. The resulting A-ring synthons were successfully applied to the synthesis of paricalcitol.
The key feature of this method is that it is flexible enough for the modification of A-ring synthons, which means that a variety of novel paricalcitol analogues modified at the A-ring should be easy to produce. Structural developments of paricalcitol based on this synthesis are currently being studied.
- Synthesis of 19-Nor-Vitamin D A-Ring Synthons via Ring-Closing Olefin Metathesis
Yu Nagai, Tomoe Tanami, Junko Abe, Hazuki Nagai, Toru Hamamizu, Kaichiro Kominato, Keisuke Iida, Kazuo Nagasawa
Asian J. Org. Chem. 2014.
DOI: 10.1002/ajoc.201402109