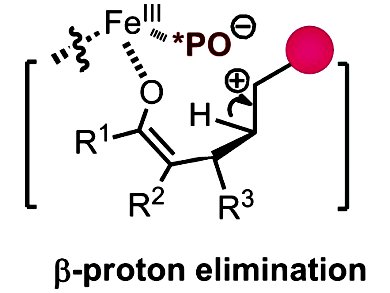
Bulk industrial procedures that involve carbocationic processes of alkenes are widely utilized. However, regarding intermolecular reactions with simple alkenes, selective C–C bond formation through a carbocationic process presents a challenge for organic synthesis. In particular, selective β-proton elimination from the carbocationic intermediate, whilst suppressing cationic polymerization and nucleophilic interception, is difficult to achieve.
Sanhong Luo, Chinese Academy of Sciences, Beijing, and co-workers have developed a strategy, in which an anionic ligand is used to tame living carbocations in Lewis-acid-catalyzed direct conjugate addition of alkenes to enones. The use of phosphate as an anionic ligand tunes the Lewis acidity of the metal center and stabilizes the developing carbocations through an ion-pair effect, thus, facilitating selective β-proton elimination and suppressing cationic olefin polymerization.
This approach provided the desired vinylation products in high yields for a wide range of substrates.
.jpg)
The researchers propose that this study will lead to the investigation of other cationic alkene functionalizations in the future.
- Taming Living Carbocations in Catalytic Direct Conjugate Addition of Simple Alkenes to α,β-Enones,
Jian Lv, Xingren Zhong, Sanzhong Luo,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201402246