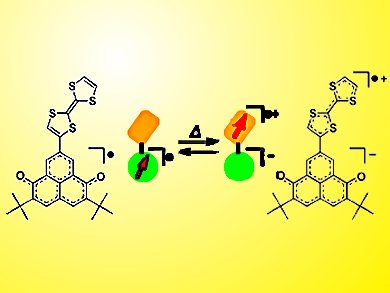
An air-stable 6-oxophenalenoxyl neutral radical with a tetrathiafulvalene moiety (TTF-6OPO) exhibits spin-center transfer with intramolecular electron transfer. This dynamic phenomenon is triggered by change of solvent or temperature and is accompanied by a continuous color change.
Yasushi Morita, Osaka University, Japan, and co-workers report that intermolecular hydrogen bonding between the neutral radical and solvent molecules is at the heart of the unprecedented phenomenon. Cyclic voltammetry shows that the redox potentials of TTF-6OPO becomes more favorable for the spin-center transfer by addition of 2,2,2-trifluoroethanol (TFE) to a benzonitrile solution.
The team used temperature-dependent cyclic voltammetry in a novel low-temperature electrochemical cell to investigate the redox potential shift with decreasing temperature in CH2Cl2–TFE solvent. Calculations revealed that the energy levels of the frontier molecular orbitals involved in the spin-center transfer are lowered by the intermolecular hydrogen-bonding interaction of the neutral radical with TFE.
- Hydrogen-Bonding Effect on Spin-Center Transfer of Tetrathiafulvalene-Linked 6-Oxophenalenoxyl Evaluated Using Temperature-Dependent Cyclic Voltammetry and Theoretical Calculations,
Shinsuke Nishida, Kozo Fukui, Yasushi Morita,
Chem. Asian J. 2013.
DOI: 10.1002/asia.201301188