Since Jean-Marie Lehn received the Novel Prize for supramolecular chemistry in 1987, chiral supramolecular catalysts have been attractive in organic synthesis. However, their practical use has been limited, as a precise synthesis of a sole designed species is difficult because of undesired aggregation of the catalysts.
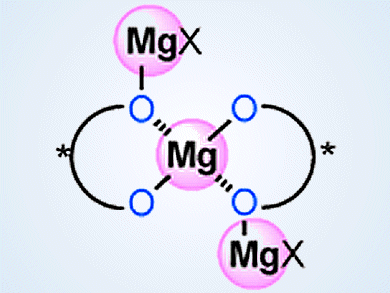
Kazuaki Ishihara and colleagues, Nagoya University, Japan, have developed highly active chiral supramolecular magnesium(II) binaphtholates based on acid–base chemistry. These binaphtholates are prepared in situ from simple, commercially available, and inexpensive Mg(II) and (R)-BINOL (1,1’-bi-2-naphthol).
These catalysts are useful for asymmetric phosphorus addition reactions, direct Mannich-type reactions, and hetero Diels-Alder reactions. Cooperative interaction between acids and bases at the optimal molar ratio triggers not only self-assembling to di- and tri-nuclear Mg(II) supramolecules but also activation of substrates and reagents effectively. The supramolecular structure and activity of these catalysts can be finely controlled by the addition of water or simple alcohols as ligands.
The team explains that this practical method to design chiral supramolecular metal catalysts may find applications in further efficient asymmetric transformations.
- Chiral Supramolecular Magnesium(II) Binaphtholate Catalysts for the Enantioselective Direct Mannich-Type Reaction and Hetero-Diels-Alder Reaction,
Manabu Hatano, Takahiro Horibe, Kenji Yamashita, Kazuaki Ishihara,
Asian J. Org. Chem. 2013.
DOI: 10.1002/ajoc.201300190