The ranking of moieties with similar chemical behavior has a fundamental role to play in supramolecular chemistry. Detailed knowledge of intermolecular interactions is crucial for the rational preparation of materials with desired structure and properties. The halogen bond (XB) is currently receiving considerable attention in supramolecular chemistry. However, as halogen bonds are relatively weak and their formation is reversible, it is particularly difficult to identify trends and patterns and to establish a hierarchy of molecular-recognition efficiency in a competitive situation.
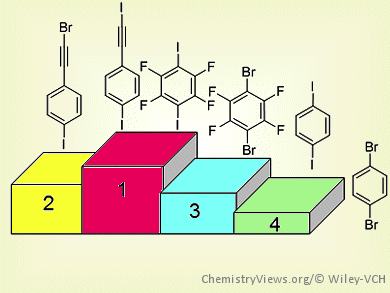
Christer Aakeröy and colleagues, Kansas State University, Manhattan, KS, USA, have explored the halogen-bond donor strength of six iodo and bromo analogues of ethynyl, perfluorinated, and phenyl-based halogen-bond donors. The team calculated the molecular electrostatic potential surfaces around each halogen-bond donor atom to give a charge-based ranking. They then allowed each XB donor to react with 21 XB acceptors through solvent-drop grinding reactions.
By combining the theoretical data with the frequency of co-crystal formation for each XB donor, the team established an XB donor ranking for six compounds.
The halogenated ethynyl moieties were extremely powerful XB donor moieties. In fact, 1-iodoethynyl-4-iodobenzene (IEIB) was shown to be comparable with the better-known XB donor 1,4-diiodotetrafluorobenzene (DITFB). Furthermore, the bromoethynyl moiety, which has been neglected as a possible XB donor, was shown to display structural capability that lies half way between DITFB and 1,4-dibromotetrafluorobenzene.
These results offer practical guidelines for synthetic crystal engineering applications and for the preparation of any chemical device that relies on an intermolecular binding event for function and performance.
- Supramolecular Hierarchy among Halogen-bond Donors,
Christer B. Aakeröy, Michele Baldrighi, John Desper, Pierangelo Metrangolo, Giuseppe Resnati,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201302162



![Unique Features of the Dinuclear Zirconocene Complex [(Cp₂Zr)₂(µ-Me)(µ-C₂Ph)]](https://www.chemistryviews.org/wp-content/uploads/2025/03/202503_Dinuclear-Zirconocene-Complex-125x94.png)