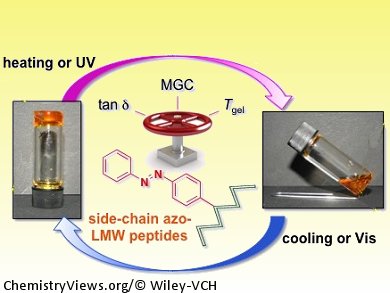
Photoresponsive supramolecular gels are useful for understanding gelation mechanisms and for advanced applications such as drug delivery and switchable devices with memory functionality. Azobenzene is the photochromic group most widely used to generate photoresponsive gels, probably due to its facile incorporation into organic structures, well-studied trans-to-cis photoisomerization, and its ability to form noncovalent interactions that stabilize some organogel structures.
Peptides are among the most popular gelating moieties, but few reports involve the creation of peptide-based, azobenzene-containing photoresponsive gels. Those that do combine the two incorporate the azobenzene unit into the peptide backbone, blocking the terminal amino moiety.
David Díaz Díaz and co-workers, CSIC-University of Zaragoza, Spain, have shown that the incorporation of azobenzene residues into the side chain of low-molecular-weight peptides can modulate their self-assembly in organic solvents, leading to the formation of stimuli responsive supramolecular organogels. The strategy allows structural flexibility since it is compatible with a free, unprotected amino terminus and allows placement of the chromophore anywhere in the peptide sequence. The driving forces for the gelation process are hydrogen-bonding and π–π interactions that can be triggered by either thermal or ultrasound stimuli.
Structure–property relationship studies of the peptide library created have shown that the presence and position of the azobenzene residue can be used to regulate the critical gelation concentration and enhance the thermal stability and mechanical strength of the gels.
- Multistimuli Responsive Supramolecular Organogels Formed by Low-Molecular-Weight Peptides Bearing Side Chain Azobenzene Moieties,
Paola Fatás, Jürgen Bachl, Stefan Oehm, Ana I. Jiménez, Carlos Cativiela, David Díaz Díaz,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201300796