The Road to Sustainable Chemical Production: Step 1, Ethylene
The various branches of the chemical industry are inextricably interdependent on the oil industry. Without oil to provide the raw material from which organic carbon can be excised and converted into the feedstock for chemical production of fine chemicals, pharmaceuticals, agrochemicals, polymers, and other materials, the industry would be a sluggish beast. As the supposedly post-industrial decades roll by, we are increasingly dependent on this raw material and yet forecasters suggest that oil reserves are dwindling and the infernal, internal combustion engine continues in its habit of burning this fossil substance as fuel.
With pressure on oil, industrious chemists are forever seeking alternative routes to the feedstocks that might be transformed into useful products. Sustainability, environmental friendliness, and renewable have become watchwords for these chemists who see the solar-powered conversion of readily available materials into plants, and the conversion of the extracts from such crops into source materials, as the way forward. For instance, rather than cracking light hydrocarbons from oil at high temperature to produce ethylene, this relatively simple two-carbon molecule can be generated from such biomass and thus displace or at least augment supplies ultimately derived from crude oil.
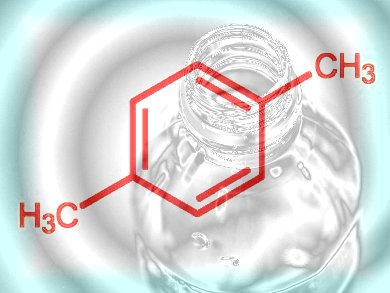
Step 2, para-Xylene
With ethylene to hand, the next step is to bump up the carbon atom count to generate more complex molecules such as para-xylene, which can then be derivatized and functionalized still further, thus acting as the next intermediate on the way to everything from polymers to pharmaceuticals with not a drop of oil spilled. Maurice Brookhart and colleagues at the University of North Carolina at Chapel Hill, USA, have developed a straightforward synthetic route from ethylene to para-xylene that represents a first small step, or perhaps a giant leap, for chemical kind that could eventually allow the industry to uncouple itself from whole-scale dependency on oil.
p-Xylene itself is one of the most important bulk chemicals: It is used to manufacture the dimethyl ester of terephthalic acid (TPA), which is then copolymerized with ethylene glycol to produce the approximately 5 million tonnes of polyethylene terephthalate (PET) every year for making plastic bottles and synthetic clothing and other fibers. 98 % of global p-xylene is used for PET production. The North Carolina team, with funding from the National Science Foundation-funded Center for Enabling New Technologies through Catalysis (CENTC), has demonstrated that they can form a trimer from ethylene to generate a six-carbon intermediate that, following dehydrogenation, undergoes a Diels–Alder reaction with another ethylene molecule to form 3,6-dimethylcyclohexene (Scheme 1). Catalytic dehydrogenation of this with platinum over alumina yields a good supply of p-xylene with minimal by-products.
.gif)
Scheme 1. Steps for conversion of ethylene into para-xylene.
The researchers point out that by replacing the conventional oil and biomass reforming processes they will not only use less energy to produce this useful product but they sidestep the inevitable isomeric side products and so preclude the need for high-temperature, complex separation steps too. They also explain that in the medium-term, ethylene is readily available from shale gas supplies. This source may not be ideal in a long-term sustainable future, but for the present it avoids the need for rapid and perhaps inefficient conversion of food crop arable land into biomass-producing land.
Step 3, Improving the Long-Term Sustainability
The ethylene to para-xylene conversion outlines by Brookhart and colleagues in the Journal of the American Chemical Society (JACS) is not yet optimized. They suggest that by avoiding the current stepwise approach they could use ethylene not only as the starting material but as the hydrogen acceptor in the dehydrogenation and so drive the reaction to even greater efficiency.
“Much remains to be done in order to make the reaction industrially attractive, e.g., higher p-xylene yield and selectivity, higher catalytic turnover rate and number, and one-pot direct conversion of ethylene at lower temperature,” says green chemist Jianliang Xiao of the University of Liverpool, UK. “The principle has been established in this publication, however. Looking ahead, a new bench, or better, continuous process may be anticipated for production of p-xylene solely based on ethylene at low temperature and with exclusive p-xylene selectivity. In the longer future, we may even see p-xylene being directly produced from bioethanol.”
James Clark, York University, UK, and Director of the York Green Chemistry Centre of Excellence, told ChemViews magazine that this, “may well be the hottest area in chemistry these days”. He points out that finding specific routes to renewable PET has, within short period of time, become one of the lead topics driven by fierce competition between the drinks manufacturers Pepsi and Coca Cola, as well as other big consumer product companies. “It can be seen as a case study and with lots of different approaches to achieve greener PET … this one is very interesting,” Clark enthuses. “It’s vital that we don’t just look at the feedstocks or products – we must look at the full life-cycle including the process – in other words we would need to assess the whole process to make sure that the process steps, converting ethylene to TPA, don’t add too much to the environmental footprint, for example, resource consumption, use of scare elements, water and energy footprints, wastes, etc.,” he adds.
- Synthesis of p-Xylene from Ethylene,
Thomas W. Lyons, Damien Guironnet, Michael Findlater, Maurice Brookhart,
J. Am. Chem. Soc. 2012.
DOI: 10.1021/ja307612b