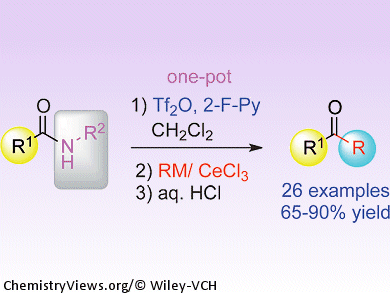
Amides are known as the least reactive class of carbonyl compounds. Their unreactive nature is sometimes useful; however, anyone who has tried to convert an amide into a different and more synthetically versatile functional group will know the frustrations encountered.
A solution to this problem is now at hand courtesy of Pei-Qiang Huang and co-workers, Xiamen University, China. Their general procedure for directly transforming secondary amides into ketones in one pot involves combining the amide with 2-fluoropyridine and trifluoromethanesulfonic anhydride to activate the carbonyl group, and then subsequent treatment with an organometallic reagent, cerium chloride, and an acid completes the deaminative alkylation. The reaction tolerates a wide range of functional groups on the substrates, and the team hopes to extend the method to produce secondary amines and ketimines from the same starting materials.
- Versatile and Direct Transformation of Secondary Amides into Ketones by Deaminative Alkylation with Organocerium Reagents,
Kai-Jiong Xiao, Ai-E Wang, Ying-Hong Huang, Pei-Qiang Huang,
Asian J. Org. Chem. 2012.
DOI: 10.1002/ajoc.201200066