Pyrroles are prevalent in many natural and non-natural products. Indeed, 3,4-disubstituted pyrroles are building blocks in tetraporphyrines and many bioactive compounds, for example, in tetrapyrrole chromophores in the plant photoreceptor phytochrome, in porphyrin, and as cyclooxygenase inhibitors and antifungal agents.
José Alemán and co-workers, Universidad Autónoma de Madrid, Spain, have broken away from the typically harsh, expensive, and multi-step pyrrole syntheses to produce pyrroles from the underrepresented 3,4-disubstituted family. A “Lego method” of enamine catalysis, followed by enamine formation, bromine substitution, NO2 elimination, and aromatization was developed to produce a wide range of 3,4-disubstituted pyrroles. This includes an N-chiral pyrrole in 96 % ee by incorporation of the amino acid L-phenylalanine. This highly modular reaction could also be used to produce bispyrroles.
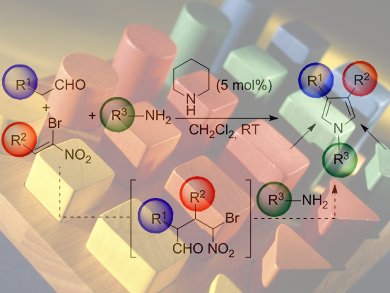
.jpg) The proposed mechanism consists of the one-pot piperidine-catalyzed reaction of an aldehyde with a bromonitroalkene to produce a 1,4-Michael intermediate, which then condenses with an amine to form an imine. A domino reaction is triggered, which affords the target pyrrole after intramolecular reactions and syn elimination of the nitro group.
The proposed mechanism consists of the one-pot piperidine-catalyzed reaction of an aldehyde with a bromonitroalkene to produce a 1,4-Michael intermediate, which then condenses with an amine to form an imine. A domino reaction is triggered, which affords the target pyrrole after intramolecular reactions and syn elimination of the nitro group.
- Modular Three-Component Organocatalytic Synthesis of 3,4-Disubstituted Pyrroles by a One-Pot Domino Reaction,
Cecilia Martín-Santos, Carlos Jarava-Barrera, Alejandro Parra, Francisco Esteban, Carmen Navarro-Ranninger, José Alemán,
ChemCatChem 2012, 4, 976–979.
DOI: 10.1002/cctc.201200104