Calixarenes, composed of phenolic units linked by methylene groups, represent one of the most widely studied classes of organic supramolecular hosts, and are described as “macrocycles with (almost) unlimited possibilities” due to their capacity for facile modification. p-Sulfonatocalix[n]-arenes (SCnAs, n = 4–8) are water solubile and biological compatibile. Possessing three-dimensional, flexible, π-rich cavities, the SCnAs family are capable of versatile complexation with a range of guest molecules, including inorganic cations, neutral molecules, organic ammonium cations, pyridiniums/viologens, and even biological or pharmaceutical molecules.
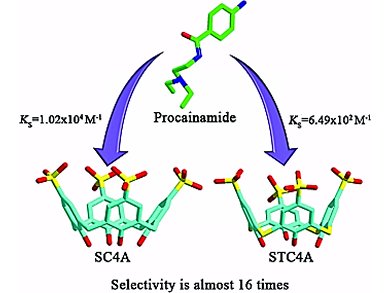
Yu Liu and colleagues, Nankai University, Tianjin, China, determined the binding geometries, abilities and thermodynamic parameters for the intermolecular complexation of three watersoluble p-sulfonatocalix[n]arenes (SCnAs), p-sulfonatocalix-[4]arene (SC4A), p-sulfonatocalix[5]arene (SC5A), and p-sulfonatothiacalix[4]arene (STC4A), with five local anesthetics (LA), procaine, tetracaine, lidocaine, dibucaine, and procainamide, by means of 1H NMR spectroscopy, X-ray crystallography, and isothermal titration calorimetry (ITC).
The obtained results show that the complexation of SCnAs with LA guests shows unappreciable guest selectivity, but regular host selectivity: SC4A > SC5A > STC4A for each guest. A large difference in the enthalpy term is responsible for the decrease in the stability constants. The binding modes depend to a certain extent on the size of the calixarene cavity and on the pH of the solution.
- Complexation of p-Sulfonatocalixarenes with Local Anaesthetics Guests: Binding Structures, Stabilities, and Thermodynamic Origins,
Qian Li, Dong-Sheng Guo, Hai Qian, Yu Liu,
Eur. J. Org. Chem. 2012.
DOI: 10.1002/ejoc.201200515