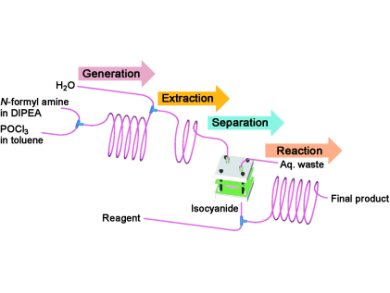
Researchers in Korea and India have developed a capillary microreactor for the in situ generation and subsequent reaction of isocyanides, which are foul-smelling and therefore undesirable to purify in a normal laboratory setting.
Dong-Pyo Kim and co-workers at the Pohang University of Science and Technology (POSTECH), Korea, used a POCl3-mediated dehydration of N-substituted formamides (that were introduced into the reactor through two different syringe pumps) to generate the desired isocyanides, which were typically formed within minutes. The reaction system was then extracted by introduction and subsequent separation with water, and the purified isocyanide transferred to a microreactor. The researchers then tested their setup with Ugi and Passerini multicomponent reactions, as well as with palladium-catalyzed oxidative coupling reactions in a batch experiments.
The authors found that with their system, not only is the handling of isocyanides avoided, but conversions can be accomplished in minutes rather than hours, and that higher yields are obtained compared to conventional synthesis.
- Odorless Isocyanide Chemistry: An Integrated Microfluidic System for a Multistep Reaction Sequence,
Siddharth Sharma, Ram Awatar Maurya, Kyoung-Ik Min, Guan-Young Jeong, and Dong-Pyo Kim,
Angew. Chem. Int. Ed. 2013.
DOI: 10.1002/anie.201303213