Metastable Form of Arsenic
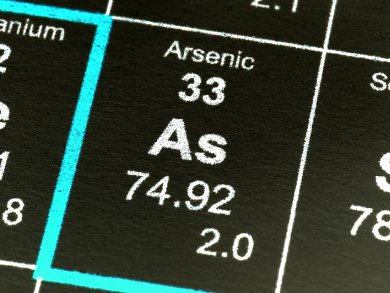
Phosphorus and arsenic are on top of each other in one group of the periodic table, so they have many similar properties. In addition to tubular forms, phosphorus is found in white, red, black, and purple structural forms. At room temperature, black phosphorus is the stable form; the others are metastable. According to textbooks, arsenic occurs in gray, yellow, and black forms. However, the existence of black arsenic, which should be analogous to black phosphorus, has never been indisputably proven. In the journal Angewandte Chemie, German researchers have now demonstrated that black arsenic is metastable in its pure form, and that it has thus far only been obtained in a form stabilized by atoms of other elements.
In their studies, a team led by Tom Nilges at the Technical University of Munich, Richard Weihrich at the University of Regensburg, and Peer Schmidt at the Lausitz University of Applied Sciences, all Germany, combined quantum chemical computations with experimental investigations of phase formation. The calculations make it possible to estimate the energetic stabilities of various structural forms of pure substances or combinations of solids, which are called solid solutions. Which phases are formed depends not only on this thermodynamic energy content, but also on the speed (kinetics) with which the individual phases form and interconvert. Metastable phases have a higher energy at defined pressures and temperatures than the stable phase. However, because a relatively high energy barrier must initially be overcome in their conversion, they only slowly convert to the stable phase, if at all.
Phase Formation and Identification
The researchers used gas-phase reactions to study phase formation. In these reactions, the solids are heated and the resulting pressure, which builds through sublimation of particles from the solid, is measured. Particles from a metastable phase enter the gas phase much more easily, so the pressure is higher than for a stable phase. When a metastable phase converts to a stable phase, the drop in pressure can be observed. It is even possible to observe pathways involving multiple different metastable intermediates.
The researchers were thus able to identify all metastable and stable phases of solid solutions of arsenic and phosphorus in all possible ratios. They were thus able to demonstrate that black arsenic is metastable in its pure form.
The results of such experiments do not only provide fundamental academic knowledge, they are also helpful in the development of targeted synthetic pathways for desirable metastable phases. This is of interest for the production of innovative materials, since metastable phases often demonstrate interesting properties. One current example of a metastable phase is an extremely hard diamond that can theoretically spontaneously convert to graphite—at room temperature—but actually never does.
- Synthesis and Identification of Metastable Compounds: Black Arsenic—Science or Fiction?
O. Osters, T. Nilges, F. Bachhuber, F. Pielnhofer, R. Weihrich, M. Schöneich, P. Schmidt,
Angew. Chem. Int. Ed. 2012.
DOI: 10.1002/anie.201106479