Interest in trivalent actinide borates stems from their potential formation in the Waste Isolation Pilot Plant (WIPP), New Mexico, USA — a geological repository for nuclear defense waste. WIPP is self-sealing, and once closed will be saturated with hydrogen and methane, making it a highly reducing environment that will favor lower oxidation states. Understanding the bonding and chemistry of these complexes is important to predict what will happen during their long-term storage.
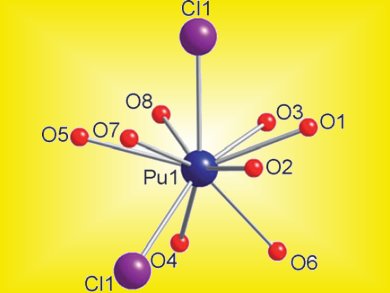
Thomas Albrecht-Schmitt and co-workers, University of Notre Dame, Indiana, USA, have used a polyborate network that is hyper-responsive to changes in the metal centers to detect changes in bonding across the actinide series when all other variables are held constant. They find that the coordination chemistry of PuIII and AmIII borates are be highly different. Different inner-sphere ligands and different coordination environments, i.e., Pu[B4O6(OH)2Cl] and Am[B9O13(OH)4]•H2O (pictured), were seen.

The differences are explained by the change in the ionic radius between neighboring actinides, ca. 0.01 Å, being enough to induce structural changes. The large difference in the average An–O bond distances between PuIII and AmIII is a consequence of different coordination environments and different structure types in PuIII and AmIII borates, as well as the substantial change in the ionic radius.
Image: © Wiley-VCH
- Bonding Changes in Plutonium(III) and Americium(III) Borates
M. J. Polinski, S. Wang, E. V. Alekseev, W. Depmeier, T. E. Albrecht-Schmitt,
Angew. Chem. Int. Ed. 2011.
DOI: 10.1002/anie.201103502