Nitrides are important materials with many applications. Compounds such as Ba3P5N10Br:Eu2+, for example, are used as white light emitting phosphors. Lithium phosphorus oxynitride can be used as a lithium-ion conductor in electrolytes. High hardness was observed in γ-P3N5.
Wolfgang Schnick and colleagues, University of Munich (LMU), Germany, have used a modified Walker-type multi-anvil press, which is capable of reaching pressures of 15 GPa and temperatures of 2800 K, to synthesize new nitride materials. The sample is placed between eight tungsten carbide anvils. These generate the high pressure when squeezed by a 1000 ton press.
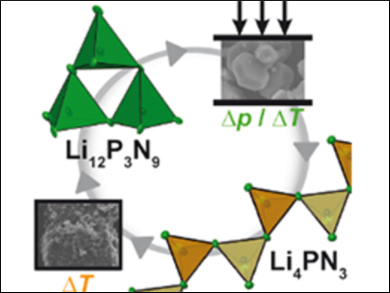
Initially, the team synthesized Li12P3N9 at relatively low pressure in sealed silica ampoules. This compound was then loaded into the Walker anvil and the pressure was raised to 9 GPa and the sample heated to 1473 K. The sample was then cooled and the pressure very slowly released over 12 hours. This process afforded a new nitride, Li4PN3 (pictured), which was characterized using X-ray crystallography. The high-pressure polymorph Li4PN3 has a novel structure made up of infinite chains of PN4 tetrahedra (pictured).
- Li12P3N9 with Non-Condensed [P3N9]12–-Rings and its High-Pressure Polymorph Li4PN3 with Infinite Chains of PN4-Tetrahedra,
Eva-Maria Bertschler, Robin Niklaus, Wolfgang Schnick,
Chem. Eur. J. 2017.
DOI: 10.1002/chem.201700979