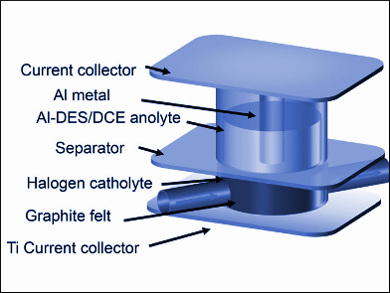
Redox-flow batteries offer separate control over energy and power and are a promising technology for stationary energy storage applications. However, a wide scale use of the technology is hindered by energy density limitations and the shortcomings of anolytes (the electrolyte around the anode). Here, the low solubility of redox species and inadequate redox potentials are problematic.
Guihua Yu, University of Texas at Austin, USA, and colleagues have developed an aluminum-based deep eutectic anolyte solvent for use in redox-flow batteries. The anolyte is prepared at room temperature from urea (a hydrogen bond donor), aluminum chloride, and the viscosity modifier 1,2-dichloroethane. The low-cost and environmentally benign anolyte is concentrated with active species and is capable of multielectron transfer processes.
When coupled with an I2 catholyte (the electrolyte around the cathode), the battery delivers an energy density of 189 Wh L–1, which is among the best energy density values reported to date. According to the researchers, microscopic metal dendrites, which are known to short circuit the electrodes of lithium batteries were not observed—suggesting that aluminum-based deep eutectic anolytes have a promising future in redox-flow battery development.
- A Sustainable Redox-Flow Battery with an Aluminum-Based, Deep-Eutectic-Solvent Anolyte,
Changkun Zhang, Yu Ding, Leyuan Zhang, Xuelan Wang, Yu Zhao, Xiaohong Zhang, Guihua Yu,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201703399