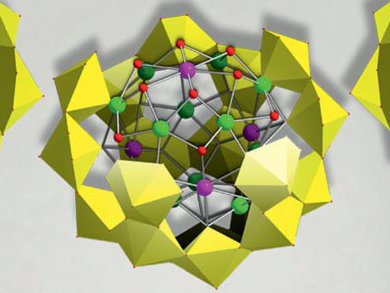
In view of the recent catastrophe in Japan, understanding the chemistry of radioactive compounds emerges as one of the most urgent scientific challenges. Uranyl peroxide compounds, as well as related trans-uranics are abundant in nuclear fuel storage pools, so their aqueous chemistry and transport properties are of interest in nuclear fuel storage and waste containment. To investigate these properties, scientists need to have pure samples in sufficient amounts.
Although a new family of actinide clusters was discovered a few years ago, the information that was revealed did not go beyond the determination of crystal structures, as the compounds were only available in small quantities. May Nyman and co-workers at Sandia National Laboratories, Albuquerque, USA, developed a reproducible, high-yield synthesis of uranyl peroxide polyoxometalates by a strategic choice of internal templating cations and anions.
A series of four U28 salts that feature different templating cations and anions was prepared on the basis of the U28 nanosphere [UO2(O2)1.5]28 that was taken as an example. The stability of these clusters in solution was demonstrated.
- The U28 Nanosphere: What’s Inside?
M. Nyman, M. A. Rodriguez, T. M. Alam,
Eur. J. Inorg. Chem. 2011.
DOI: 10.1002/ejic.201001355