To use artificial photosynthesis to provide fuels from water and sunlight, fast molecular catalysts that can efficiently perform both the reduction and oxidation of water are required. In the particular case of metal complexes that catalyze O2 evolution, the rational design of the catalysts has led to an increase in the reaction rate by a factor of 104 during the last decade. One of the main challenges is the immobilization of these molecular catalysts onto conductive surfaces without losing the intrinsic activity of the catalyst.
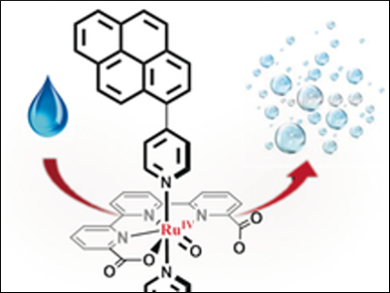
Pascal Blondeau and Cyril Godard, Universitat Rovira i Virgili, Tarragona, Spain, Xavier Sala and Antoni Llobet, Universitat Autònoma de Barcelona, Cerdanyola del Vallès, Spain, and colleagues have prepared the ruthenium-based water oxidation catalyst precursors [Ru(tda)(Li)2] (tda2− = [2,2′:6′,2′′-terpyridine]-6,6′′-dicarboxylato; L1 = 4-(pyren-1-yl)-N-(pyridin-4-ylmethyl)butanamide, L2 = 4-(pyren-1-yl)pyridine)). Both complexes contain a pyrene group allowing efficiently anchoring via π interactions on multi-walled carbon nanotubes (MWCNT) on glassy carbon electrodes without interfering with the active center of the Ru catalyst. These hybrid solid state materials are very stable molecular water-oxidation anodes capable of carrying out more than a million turnover numbers.
The ruthenium catalyst contains a strategically situated dangling carboxylate group that assist the water oxidation catalysis and enhances turnover frequencies up to 8000 s–1, with high catalyst stability. According to the researcherss, the anode represents a step forward in the field of solar fuel technology.
- A Million Turnover Molecular Anode for Catalytic Water Oxidation,
Jordi Creus, Roc Matheu, Itziar Peñafiel, Dooshaye Moonshiram, Pascal Blondeau, Jordi Benet-Buchholz, Jordi García-Antón, Xavier Sala, Cyril Godard, Antoni Llobet,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201609167




Very novel and meaningful work, it is worth learning