Enantiopure aminocyclopropanes are important components in biologically active natural products and pharmaceuticals. The most straightforward and atom-efficient route for the construction of chiral aminocyclopropane units is the asymmetric hydroamination of substituted cyclopropenes with amines. However, this transformation is difficult because of the easy ring-opening of the highly strained cyclic skeleton.
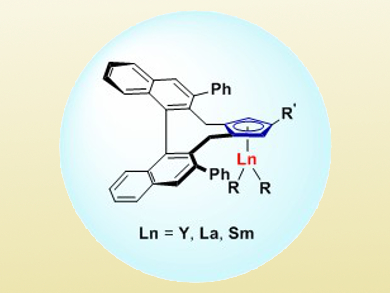
Zhaomin Hou and colleagues, RIKEN, Saitama, Japan, have developed an enantioselective hydroamination of substituted cyclopropenes (e.g., 3-methyl-3-phenylcyclopropene) with various amines. They used chiral half-sandwich rare-earth-metal catalysts with yttrium, lanthanum, and samarium (pictured).
This method constitutes a 100 % atom-efficient route for the synthesis of a variety of chiral aminocyclopropane derivatives in high yields (up to 96 %) and excellent stereoselectivity (up to >20:1 d.r. and 99 % ee) under mild reaction conditions (25 °C). According to the researchers, these complexes could be a platform for asymmetric catalysis beyond hydroamination.
- Synthesis of Chiral Aminocyclopropanes by Rare-Earth-Metal-Catalyzed Cyclopropene Hydroamination,
Huai-Long Teng, Yong Luo, Baoli Wang, Liang Zhang, Masayoshi Nishiura, Zhaomin Hou,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201609853