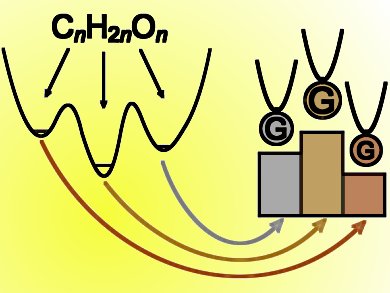
For the carbohydrate formula CnH2nOn the lowest-energy species are not necessarily carbohydrates. Taking into account zero-point energy, a systematic treatment of CnH2nOn for n = 1–4 and beyond reveals that small-molecule aggregates, such as the complex of CH4 with CO2 for n = 2, are actually the winners over larger contiguous molecules. These winning structures can be called Guinness molecules, and the winners for the carbohydrate formula show many parallels with products of carbohydrate fermentation.
The fact that the small-molecule aggregates are favored over larger contiguous molecules also helps to explain why some molecules, such as acetic acid, are found only in low abundance in interstellar space. They also provide a model for thinking about atmospheric CO2 capture, as CO2 complexes are among many of the lowest-energy species.
The search for Guinness molecules by computational treatment stemmed from a research-oriented teaching and learning project under the guidance of Professor Martin A. Suhm, with co-supervision from Ph.D. student Jonas Altnöder and active contribution of ten motivated first-year chemistry bachelor students at the University of Göttingen, Germany. The group approached each formula with basic assumptions on the bonding in the systems and worked out full geometry optimizations using appropriate basis sets.

This computational approach can also be applied to a range of non-carbohydrate formulas. Teachers and students are highly encouraged to choose further molecular formulas and find new Guinness molecules. What about saturated hydrocarbons or even carbon allotropes? A rewarding search relies on combinatorial reasoning, available experimental/computational information, and a certain degree of chemical intuition and understanding of valence rules.
- The Guinness Molecules for the Carbohydrate Formula,
Jonas Altnöder, Kerstin Krüger, Dmitriy Borodin, Lennart Reuter, Darius Rohleder, Fabian Hecker, Roland A. Schulz, Xuan T. Nguyen, Helen Preiß, Marco Eckhoff, Marcel Levien, Martin A. Suhm,
Chem. Rec. 2014.
DOI: 10.1002/tcr.201402059
Find more world records from all branches of chemistry on the Records and Challenges platform of The Chemical Record, under www.tcr.wiley-vch.de/records.